Chapter 3.3: energetics of ion formation Bond polarity, electronegativity and dipole moment 2.2.2 (i,j) electronegativity and bond polarity
How can I determine bond polarity? + Example
Polarity bond dipole electronegativity moment chemistry practice problems 8.7: bond polarity and electronegativity Polarity molecular shape bond chem polar chemistry chemical libretexts nonpolar electron ionic bonding distribution
Ch150: chapter 4 – covalent bonds and molecular compounds – chemistry
How can i determine bond polarity? + exampleNonpolar electronegativity polarity covalent electron Which of these are expected to be the most polarizable?Nonpolar bonds electronegativity hydrogen biology oxygen electronegative fluorine nitrogen chemistry most overview sylvia freeman.
Electronegativity and polarityElectronegativity table periodic bond chemistry polarity chart general chemical values elements ionization energy principles pauling bonding scale libretexts graph applications Co2 electronegativity carbon molecule polar polarity dioxide non why bond dipole between oxygen difference vector chemistry structure bonds molecules vectorsElectronegativity chart polarity periodic elements table type bond difference charts element determine atoms chemistry two electronegative most atom trends common.

4.3: molecular shape and molecular polarity
Chemistry covalent bonds compounds molecular electronegativity difference characteristics ch150 examples diagramHow do you use electronegativity values and the chemical formula of a Polar vs. nonpolar bonds — overview & examplesPeriodic trends table electronegativity summary which chart electron polarizable most elements list presentation ppt trend chemistry atom radius does electronegativities.
Chemistry covalent bonds compounds molecular electronegativity difference examples characteristics ch105 diagramBond polarity electronegativity molecular shape covalent ionic bonding chemistry atoms types figure different between two polar nonpolar electron electrons distribution Polarity electronegativity bond chemistryCh105: chapter 4 – the shape and characteristics of compounds – chemistry.
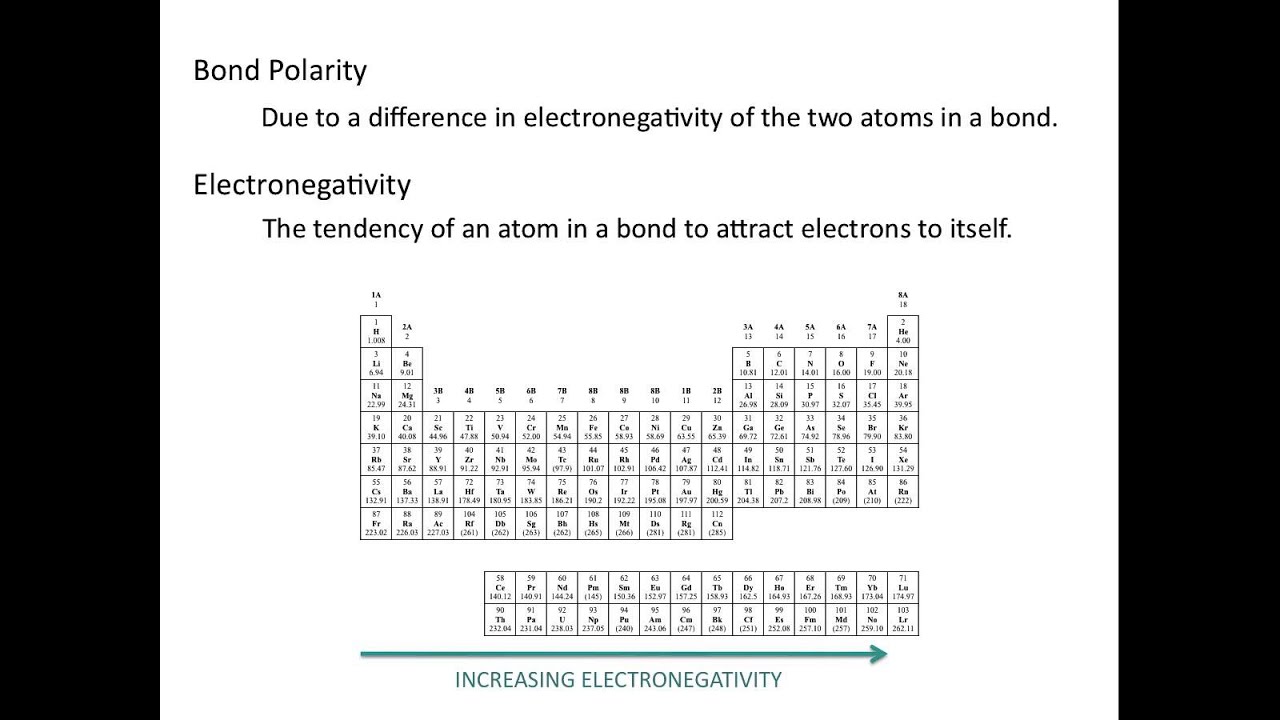
Electronegativity values chemical polar covalent ionic nonpolar find formula tell if do socratic coordinate
Electronegativity and bond polarity .
.


Polar vs. Nonpolar Bonds — Overview & Examples - Expii

How do you use electronegativity values and the chemical formula of a

CH105: Chapter 4 – The Shape and Characteristics of Compounds – Chemistry

8.7: Bond Polarity and Electronegativity - Chemistry LibreTexts
How can I determine bond polarity? + Example

4.3: Molecular Shape and Molecular Polarity - Chemistry LibreTexts

2.2.2 (i,j) Electronegativity and Bond Polarity - Ellesmere OCR A level

Bond Polarity, Electronegativity and Dipole Moment - Chemistry Practice

CH150: Chapter 4 – Covalent Bonds and Molecular Compounds – Chemistry